The Advantages of Non-Contact Dispensing
Key Takeaways: Non-contact dispensing for diagnostics and IVD reduces contamination risk, eliminates disposable tip waste, and supports ESG goals by...
3 min read
Stephanie Moreau & Sean Madden : November 25, 2025

Key Takeaways:
Biological samples can contaminate pump internals, reducing accuracy, causing biofouling, and requiring costly, time-consuming cleaning.
Traditional peristaltic pumps isolate samples but lose precision and demand frequent tubing replacement, increasing long-term cost.
Fluid Metering’s contamination-free sample dispensing uses barrier media—buffer, high-purity water, or air—to keep samples completely isolated from the pump.
This approach preserves precision, prevents cross-contamination, and reduces maintenance, delivering a more reliable and cost-effective solution for medical and diagnostic instruments.
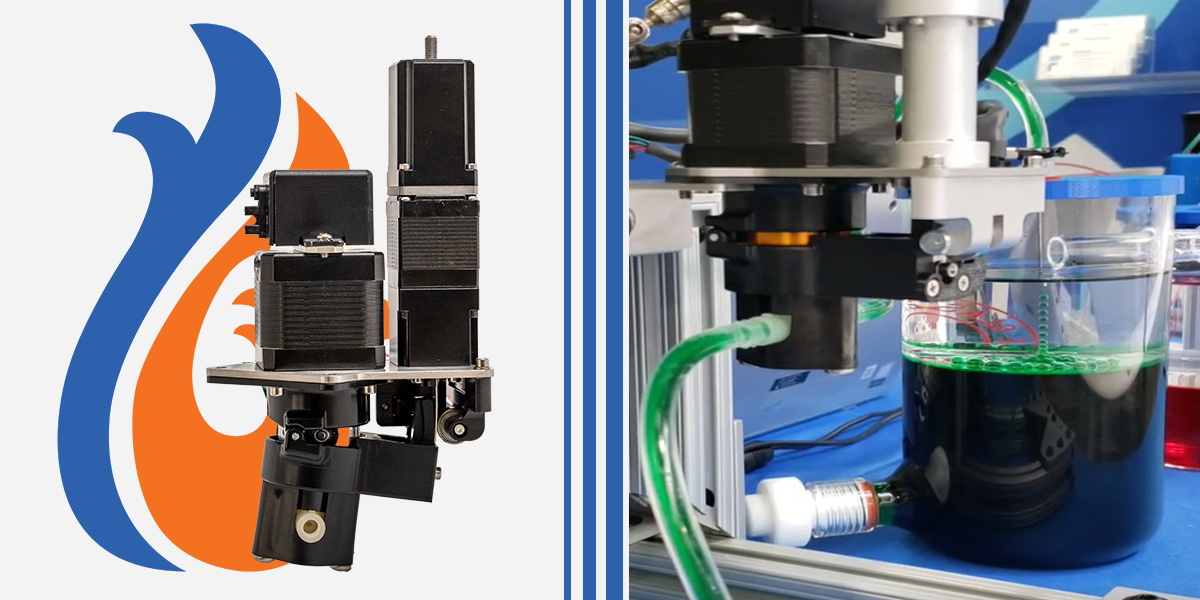
In medical testing and laboratories, pumps need to move biological samples, but those same samples – blood, serum, cells, proteins – can contaminate the pump's internal parts. Peristaltic pumps can be used but they often lose precision and accuracy over time and is expensive to replace the tubing needed. Fluid Metering pumps offer a cost effective and reliable solution by using a protective barrier media, called contamination-free sample dispensing.
The Contamination Problem
When pumps directly contact biological samples the consequences are significant. Residual material contaminates subsequent dispenses, proteins adhere to internal surfaces degrading performance, and cleaning requires aggressive protocols or complete disassembly. Biofouling, cross-contamination, and infection control concerns create both operational headaches and regulatory burdens for medical device manufacturers.
How Barrier Medias Solve the Problem
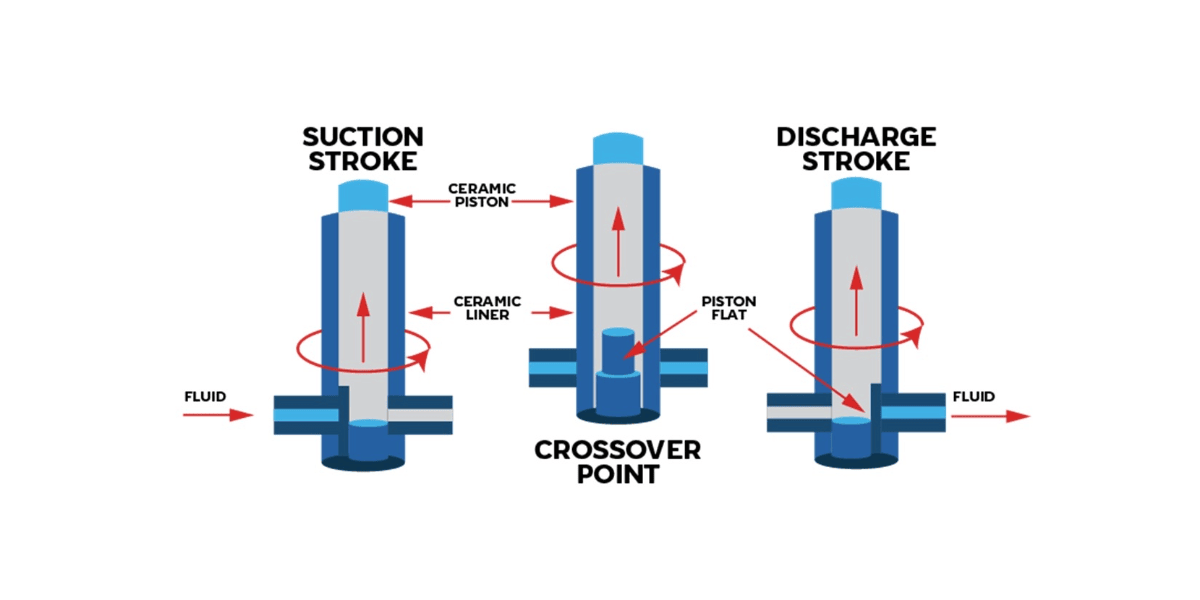
Contamination-free sample dispensing elegantly sidesteps these challenges through a three-step process that keeps biological fluids completely isolated from pump internals:
Step 1: Prime with Barrier Media - The pump is filled with the chosen barrier media that will protect the pump internals from biological samples. Options include:
Step 2: Create Additional Barrier (Air Barrier Technique Only) - When using the air barrier approach, a precise volume of air is aspirated into the fluid path, creating a compressible cushion between the priming fluid inside the pump and the biological sample. This step provides volume flexibility and easy visual confirmation of barrier integrity.
Step 3: Aspirate and Dispense - The biological sample is drawn into the tubing. During dispensing, the priming fluid pushes the sample forward (or pushes the air which then pushes the sample in air barrier applications), delivering it with precision while never contacting the pump's internal components.
Choosing the Right Barrier Approach
The selection of which barrier approach depends on specific application requirements:
Air Technique (prime with buffer/water + aspirate air) excels when:
High Purity Water Barriers (prime with high purity water only) are preferred when:
Buffer Solution Barriers (prime with buffer only) work best when:
Why FMI Pumps Excel at Barrier Media Protection
Fluid Metering’s pump technology is uniquely suited for this contamination-prevention approach regardless of barrier media choice. The positive displacement design provides precise control over buffer volume, air volume, and sample volume, ensuring the protective barrier remains intact throughout every dispense cycle.
Unlike peristaltic or diaphragm pumps, FMI's sealed piston design handles both liquid barrier approaches and air barrier techniques predictably and repeatably. For liquid barriers, the incompressible priming fluid provides direct, consistent force transmission. For air barrier applications, the pump manages the compressible air barrier without unwanted migration. The smooth, continuous piston motion ensures reliable sample delivery in both approaches, without retention or dead volume issues.
With ceramic and PTFE wetted materials, FMI pumps accommodate virtually any buffer solution while remaining chemically inert. This versatility allows system designers to optimize both internal buffer and barrier media selection for their specific application without material compatibility constraints.
Real-World Impact
Medical device manufacturers are implementing contamination-free sample dispensing with FMI pumps across diverse applications: clinical analyzers handling blood and serum, genomics workstations dispensing PCR reagents, immunoassay platforms moving antibody solutions, cell culture automation systems, bioprocessing equipment, and veterinary diagnostic instruments. In each case, the barrier media—whether air, high purity water, or specialized buffer—enables precise biological sample handling without the contamination risks, maintenance burdens, or performance degradation associated with direct pump-sample contact.
Beyond Contamination Control
The contamination-free sample dispensing approach delivers system-level advantages that impact the bottom line. Service intervals extend dramatically when biological materials never touch pump internals. Cleaning simplifies to external tubing and dispense tip only, not the pump mechanism. The same tubing set can handle multiple samples with simple rinses, reducing disposables costs. Regulatory validation becomes less complex without pump-sample contact concerns.
Most importantly, pumps maintain factory-fresh performance characteristics even after thousands of biological sample dispenses, translating to lower cost of ownership through reduced maintenance labor, fewer replacement parts, and minimal downtime.
Flexible Protection for Every Application
Contamination-free sample dispensing represents a fundamental shift in biological fluid dispensing philosophy. By embracing protective barriers—whether through liquid priming media or air—rather than treating contamination as inevitable, instrument designers can deliver the precision medical diagnostics demand while keeping pump mechanisms pristine.
Whether using high purity water or buffers as direct liquid barriers, or implementing air slug techniques for added flexibility, the barrier approach adapts to each application's unique requirements. Fluid Metering’s pump technology makes this approach both practical and reliable, enabling a new generation of medical devices that handle sensitive biological materials without compromise.
Key Takeaways: Non-contact dispensing for diagnostics and IVD reduces contamination risk, eliminates disposable tip waste, and supports ESG goals by...
Key Takeaways: Fluid Metering’s CeramPump® technology for OEM fluid control supports rinse, aspirate, sample, dispense, and dilute functions—all in...

1 min read
Key Takeaways: Clinical chemistry and immunoassay analyzers require microliter-level precision, fast fluid handling, and contamination...